Caffeine attenuates spermatogenic disorders in mice with induced chronic scrotal hyperthermia
Article information
Abstract
Objective
Chronic scrotal hyperthermia (SHT) can lead to serious disorders of the male reproductive system, with oxidative stress playing a key role in the onset of these dysfunctions. Thus, we evaluated the impact of caffeine, a potent antioxidant, on cellular and tissue disorders in mice with chronic SHT.
Methods
In this experimental study, 56 adult male NMRI mice were allocated into seven equal groups. Apart from the non-treated control group, all were exposed to heat stress. Two groups, termed “preventive” and “curative,” were orally administered caffeine. The preventive mice began receiving caffeine immediately prior to heat exposure, while for the curative group, a caffeine regimen was initiated 15 consecutive days following cessation of heat exposure. Each treated group was subdivided based on pairing with a positive control (Pre/curative [Cur]+PC) or a vehicle (Pre/Cur+vehicle). Upon conclusion of the study, we assessed sperm characteristics, testosterone levels, stereological parameters, apoptosis, antioxidant and oxidant levels, and molecular markers.
Results
Sperm parameters, testosterone levels, stereological parameters, biochemical factors (excluding malondialdehyde [MDA]), and c-kit gene expression were significantly elevated in the preventive and curative groups, especially the former, relative to the other groups. Conversely, expression levels of the heat shock protein 72 (HSP72) and nuclear factor kappa beta (NF-κβ) genes, MDA levels, and apoptotic cell density were markedly lower in both caffeine-treated groups relative to the other groups, with more pronounced differences observed in the preventive group.
Conclusion
Overall, caffeine attenuated cellular and molecular abnormalities induced by heat stress in the testis, particularly in the mice treated under the preventive condition.
Introduction
Infertility is a multifaceted disorder with substantial medical, psychological, and economic implications [1]. It is particularly prevalent due to male factors, with the environment playing a major role [2,3]. In various mammalian species, a relatively low temperature is necessary for the normal progression of spermatogenesis and sperm fertility [4,5]. Research has demonstrated that the scrotal temperature in most mammals is 2 to 8 °C lower than the internal body temperature [4]. Heat damage to the testes is a primary cause of infertility in men and most mammals [5,6]. Even brief exposure of the testes to heat can halt spermatogenesis, although the induced damage is typically reversible within approximately 73 days [7].
Apoptosis of testicular germ cells plays a crucial role in both testicular development and the destruction of germ cells under physiological and pathological conditions [8,9]. Prior research has demonstrated that hyperthermia triggers cell death in the testis, leading to a noticeable death of primary spermatocytes and spermatogonia [10-12]. Various drugs, with distinct mechanisms, have been employed to mitigate the impact of heat stress on spermatogenic cells in testicular tissue. Concurrently, the use of antioxidants to counteract these adverse effects has been explored [13,14].
Caffeine (1,3,7-trimethylxanthine) is an alkaloid found in the leaves, seeds, or nuts of numerous plants. It is consumed daily by a large number of people worldwide, primarily due to its presence in tea and coffee [15]. Research has demonstrated that in testicular germ cells, uncoupling protein 2 reduces apoptosis caused by hyperthermia [16]. Caffeine has been found to upregulate the expression of this factor [17]. During hyperthermia, endonuclease enzymes are activated, leading to the fragmentation of DNA in germ cells into low-molecular-weight chains. This endonuclease-induced DNA cleavage is recognized as a sign of apoptosis in testicular germ cells, particularly in pachytene spermatocytes and spermatids [9,18]. One potential mechanism through which hyperthermia impacts cells involves heat shock proteins [19]. These proteins are found in various cellular species under normal conditions, but their levels markedly rise under hyperthermic conditions [19-21]. Heat shock proteins prevent other proteins from denaturing and accumulating in cells that are exposed to changes such as high temperature, inflammatory mediators, or infection [21-23]. As the temperature in the testicular tissue rises, the level of lipid peroxidation in germ cells increases, and antioxidant enzyme activity is concurrently compromised [24]. Antioxidant enzymes like catalase (CAT) have been shown to provide significant protection against apoptosis induced by heat or oxidative stress by neutralizing hydrogen peroxide [25-28]. Given the pathogenesis of testicular hyperthermia and the potent free radical scavenging capacity of caffeine, we conducted this study to explore the clinical benefits and molecular mechanisms underlying the use of caffeine in a chronic scrotal hyperthermia (SHT) model.
Methods
1. Ethical approval
The Ethics Committee of Sabzevar University of Medical Sciences in Sabzevar, Iran approved all experimental protocols (Ethic No.: IR.MEDSAB.REC.1400.070). Animals were handled in accordance with the protocol of animal management and welfare of the University of Helsinki, Finland. The animals were housed in standard laboratory hutches and given unrestricted access to rodent food and drinking water. All procedures were conducted in accordance with relevant guidelines and regulations. The study adhered to the Animal Research: Reporting of In Vivo Experiments guidelines. Unless otherwise specified, all materials used in this study were procured from Sigma-Aldrich.
2. Animals and experimental design
In this experimental study, a total of 56 adult male NMRI mice (each weighing 25 to 30 g) were obtained from the Pasteur Institute in Mazandaran, Iran. The animals were housed and fed according to the ethical standards of Sabzevar University of Medical Sciences. The mice were divided into seven groups (with n=8 for each group). One group was an untreated control. The remaining mice underwent SHT induction and were randomly assigned to two primary groups: preventive and curative. Each of these was further divided into three subgroups. The preventive subgroups included preventive, in which mice received caffeine starting 2 hours prior to SHT induction and continuing until the end of the 5th week; the positive control for the preventive group (Pre+PC), which did not receive any treatment; and Pre+vehicle, which received distilled water (used as a caffeine solvent) in the same volume and at the same times as the preventive group. The curative subgroups included curative, which received caffeine daily for 15 days following the SHT induction period; the positive control for the curative group (Cur+PC), which did not receive any treatment; and Cur+vehicle, which received distilled water in the same volume and at the same times as the curative group. Given that the damaging effects of heat stress on sperm parameters and DNA become apparent 14 days after the stress, we set the treatment duration at 15 days [29,30]. Figure 1 illustrates the overall study timeline for the experimental groups. We selected the caffeine dose (10 mg/kg) based on previous studies and our pilot experiments [31]. Caffeine was administered via oral gavage, dissolved in 20 µL of distilled water. At the end of the experiment, the animals were anesthetized with an intraperitoneal injection of ketamine and xylazine. First, blood was collected via cardiac puncture to evaluate testosterone levels. Immediately afterward, the scrotum was opened, and the tails of the epididymis were isolated for semen analysis. Finally, both testicular tissues were harvested. The right testicular tissue was used for stereological and immunohistochemical evaluation, while the left was utilized for biochemical and molecular testing.
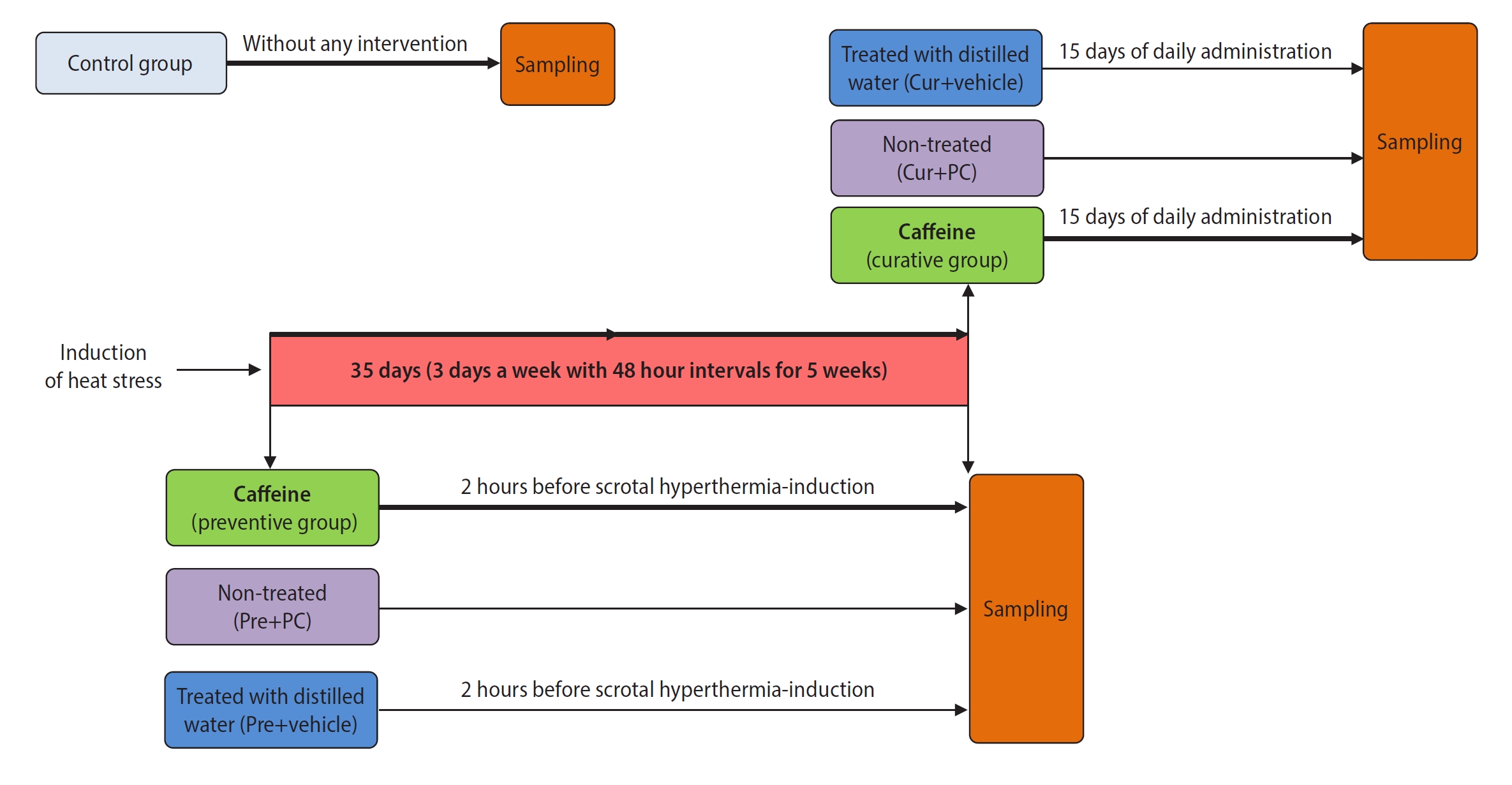
Overall study timeline for the experimental groups. This schematic shows the time points of scrotal hyperthermia induction, caffeine administration, and sampling. Boxes of the same color indicate similarities in the type of intervention between subgroups of the preventive and curative treatment groups. Cur, curative; PC, positive control.
3. SHT model
Chronic SHT was induced in accordance with the study conducted by Ziaeipour et al. [32]. To achieve this, the animals were anesthetized through the intraperitoneal administration of ketamine (100 mg/kg) and xylazine (5 mg/kg). Following the induction of SHT, the lower third of the mouse, encompassing the scrotum, tail, and legs, was submerged in a water bath at 43 °C for 20 minutes on alternate days over a period of 5 weeks.
4. Testosterone evaluation
The collected blood was subjected to centrifugation at 2,500 ×g for 15 minutes, resulting in the isolation of blood serum. Testosterone levels were determined using an enzyme-linked immunoassay kit. For this procedure, the samples were introduced into 96-well microplates, and the absorbance was measured at 640 nm using a microplate reader (Biocompare; Agilent Technologies) [33]. All samples were analyzed in triplicate.
5. Semen analysis
After the semen samples were isolated from the tails of the epididymis, they were immediately transferred to 1 mL of Ham F-10 medium and incubated for 20 minutes at 37 °C. To obtain the sperm count, sperm suspensions were pipetted and filtered through 80-μm nylon mesh to eliminate tissue fragments. A 0.05-mL aliquot from the 1 mL of sperm suspension was diluted with 1:40 phosphate-buffered saline (PBS, pH 7.2) and thoroughly mixed. A sample of this diluted sperm suspension was then placed into a counting chamber (Sperm Meter; Sperm Processor) after discarding the initial few drops of suspension. The total sperm count was determined by counting the sperm in eight 1-mm2 squares and multiplying the result by 5×106 to estimate the total number of sperm per epididymis [34]. To assess sperm motility, 10 μL of the sample was separated and examined under an inverted microscope. Sperm viability was evaluated using eosin-nigrosin staining. Additionally, sperm morphology was assessed using a Diff-Quik staining kit [35].
6. Histological and stereological evaluation
At the conclusion of the study, each animal was sacrificed, and the right testicle was extracted and preserved in 10% formalin. Following tissue processing and paraffin embedding, 10 evenly spaced sections were selected and stained with hematoxylin and eosin. The Johnsen test was employed for histological evaluation of the tissue structure [33]. Stereological evaluations included the total volume of testicular tissue, the length density of seminiferous tubules, and the numerical densities of various cells, including spermatogonia, primary spermatocytes, spermatids, Sertoli cells, and Leydig cells [36,37]. To evaluate the tissue structure and total volume, 5-μm-thick sections were prepared, while for the other measurements, 20-μm-thick sections were used.
1) Testicular tissue structure
After the selection and staining of tissue sections, photographs were taken of five distinct areas in each section at a magnification of ×400. These areas included the upper and lower right and left corners, as well as the center of the tissue. Subsequently, the Johnsen scoring system was utilized to evaluate the degree of tissue structure damage. This scoring system assigns a minimum score of 1 and a maximum score of 10, which correspond respectively to the absence of any epithelium in the seminiferous tubules and the completion of spermatogenesis along with the presence of numerous spermatozoa [33].
2) Total testicular volume
The Cavalieri method was employed for the determination of volume. To this end, all 10 sections obtained from each mouse were superimposed onto a grid of points. Subsequently, to ascertain the volume, all points overlaying each section were counted and analyzed using the following equation.
Here, ΣP represents the total number of points counted, a/p (in mm2) indicates the area associated with each square formed between four points, and t (in mm) denotes the distance between the selected sections, as perceived.
3) Numerical density of cells
To determine the numerical densities (Nv values) of spermatogonia, primary spermatocytes, spermatids, Sertoli cells, and Leydig cells, we employed the optical dissector method. This involved using a standard probe, positioned on a monitor connected to a microscope. We examined tissue sections at ×400 magnification and calculated the numerical density using the following formula.
Here, ΣQ denotes the total number of nuclei, Σp indicates the total number of counted frames, h (in µm) represents the height of the dissector, a/f (in mm2) indicates the frame area, t (in µm) denotes the real sectional thickness, and BA (in µm) represents the block advance of the microtome, which was set at 20 μm.
4) Length density of seminiferous tubules
The formula
7. Immunostaining for apoptosis
Apoptosis was evaluated through immunostaining against the caspase-3 protein. For this procedure, 10 tissue sections from each mouse were chosen, as outlined in the description of stereological evaluation. These sections were then deparaffinized and exposed to goat normal serum to block nonspecific sites. Subsequently, an anti-caspase 3 rabbit polyclonal antibody (1:100 in PBS; Abcam ab4051; Abcam) was applied and left to incubate overnight at 4 °C. Following this, the sections were rinsed with PBS and subjected to a secondary antibody (goat anti-rabbit immunoglobulin G; Abcam). Diaminobenzidine tetrahydrochloride was then added to the sections for 5 minutes to identify cells with a positive reaction. After dehydration, the samples were mounted, and five images of each section were captured [38,39]. Densitometry, using MacBiophotonics ImageJ software version 6 (National Institutes of Health), was employed to calculate the average level of apoptosis.
8. Biochemical evaluation
The harvested samples were washed with sterile PBS to eliminate excess tissue residue, after which they were immediately frozen at −80 °C for subsequent analysis. The biochemical status of the injury site was evaluated by investigating the concentrations of glutathione (GSH), superoxide dismutase (SOD), and CAT as antioxidant biomarkers and malondialdehyde (MDA) as an oxidant factor. All biomarkers were quantified using the method described by Nasiry et al. [33]. In brief, trichloroacetic acid and pyrogallol were utilized to measure the respective levels of GSH and SOD, and their absorption levels were documented via spectrophotometry at the respective wavelengths of 420 and 412 nm. The CAT level was measured using hydrogen peroxide in conjunction with a phosphate buffer, and its absorbance was recorded at a wavelength of 245 nm. Finally, the concentration of MDA was determined using thiobarbituric acid, and its absorbance was documented at a wavelength of 520 nm.
9. Gene expression analysis
Quantitative real-time polymerase chain reaction was employed to quantify the gene expression of the inflammatory cytokine nuclear factor kappa beta (NF-κβ), as well as proto-oncogene c-KIT (c-kit) and heat shock protein 72 (HSP72), which are involved in spermatogonial differentiation, proliferation, and resistance to oxidative stress. In this procedure, tissue samples were promptly homogenized following collection, and total RNA was subsequently extracted using a total RNA extraction kit (Yekta-tajhiz). The complementary DNA (cDNA) MultiScribe kit (Thermo Fisher) was utilized to prepare cDNA [40,41]. Spectrophotometry was used to evaluate the cDNA, and the results indicated that the absorbance ratio for all samples, between the wavelengths of 260 and 280 nm, ranged from approximately 1.8 to 2.01. Ultimately, the levels of gene expression were ascertained using a real-time polymerase chain reaction instrument (StepOne; Applied Biosystems). The sequences of the primers used in this process are presented in Table 1. Furthermore, β-actin was used as an internal control.
10. Statistical analysis
All data were analyzed using SPSS version 21 (IBM Corp.). The Kolmogorov-Smirnov test was employed to assess the distribution of the data, which were found to be normally distributed. To examine the relationship between groups, one-way analysis of variance and the Tukey post hoc test were utilized. Quantitative data are presented as the mean±standard deviation. A p-value of less than 0.05 was considered to indicate statistical significance.
Results
In all cases, the control mice demonstrated superior results to the other groups. Therefore, we present a comparison of the results among the groups that underwent SHT induction.
1. Administration of caffeine improved sperm characteristics in mice with induced chronic SHT
Figure 2 presents the results regarding sperm characteristics. The preventive and curative groups significantly outperformed the other groups in all measured sperm characteristics, including count, viability, motility, and morphology (all p<0.0001). Furthermore, when comparing sperm characteristics between the caffeine-treated groups, the preventive group exhibited significantly higher sperm count (p<0.001), viability (p<0.001), motility (p<0.05), and morphology (p<0.001) than the curative group.
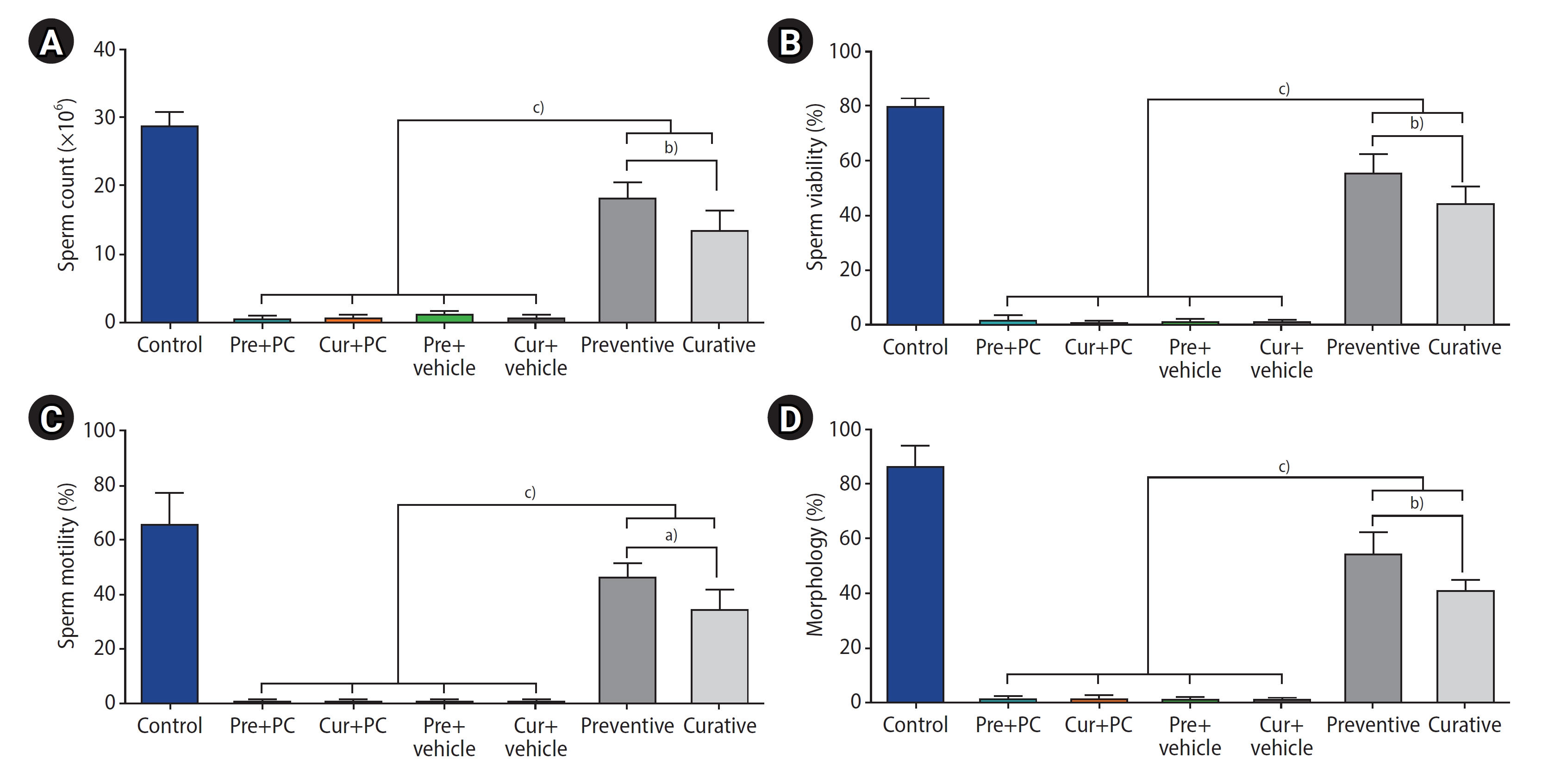
Effects of caffeine on sperm characteristics in mice with induced chronic scrotal hyperthermia. (A) Sperm count, (B) viability, (C) motility, and (D) morphology were compared across the study groups. Data are presented as mean±standard deviation. Pre, preventive; PC, positive control; Cur, curative. a)p<0.05; b)p<0.001; c)p<0.0001.
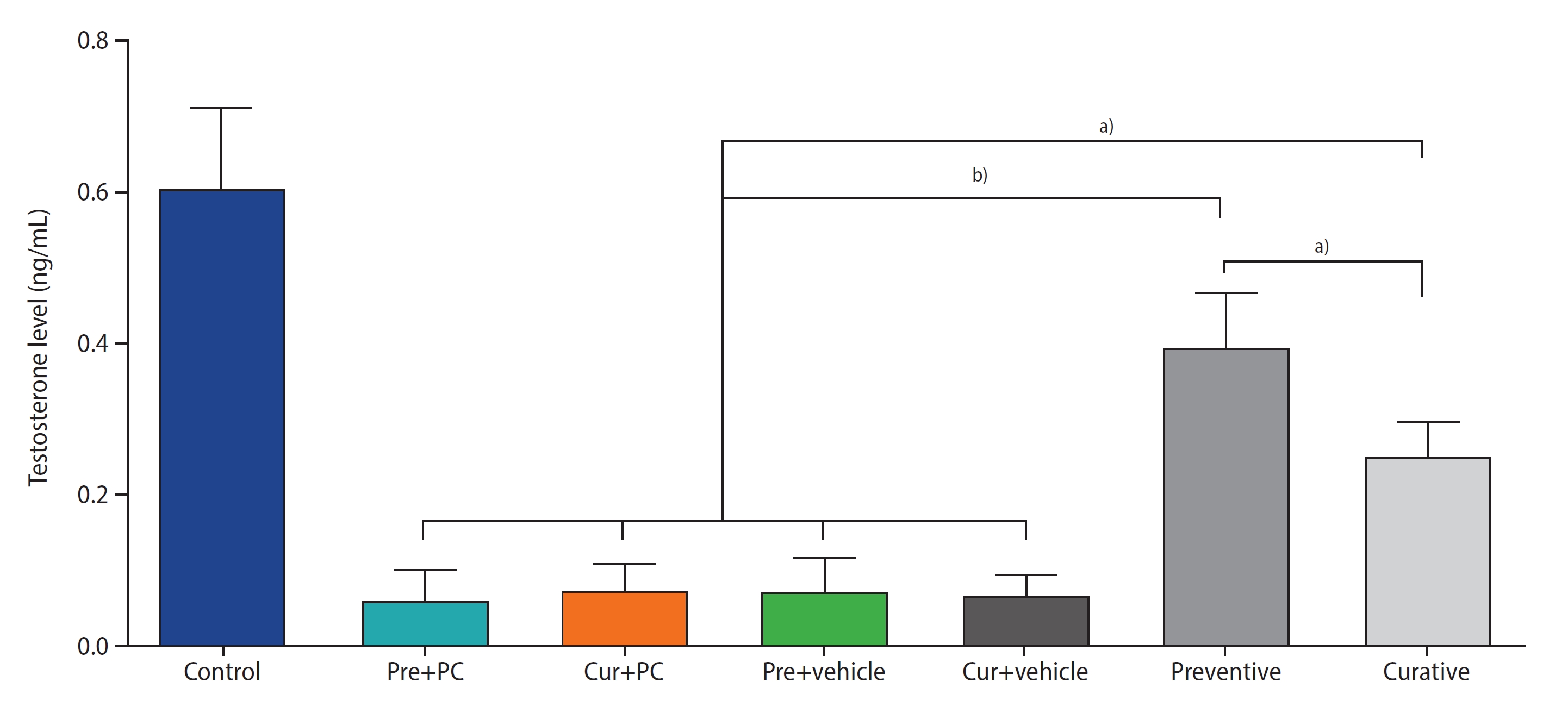
2. Administration of caffeine increased testosterone levels in mice with induced chronic SHT
Figure 3 presents the results regarding testosterone levels. Both the preventive (all p<0.0001) and curative (all p<0.01) groups exhibited significantly higher testosterone levels than the other groups. Moreover, the testosterone level in the preventive group was significantly greater than that in the curative group (p<0.01).
3. Administration of caffeine prevented structural damage and improved stereological parameters in mice with induced chronic SHT
1) Testicular structure
Under the Johnsen scoring system, the mean±standard deviation for the control, Pre+PC, Cur+PC, Pre+vehicle, Cur+vehicle, preventive, and curative groups were 9.68±0.32, 2.35±0.49, 2.7±0.61, 2.23±0.48, 2.66±0.7, 7.54±1.57, and 5.09±1.29, respectively. The mean Johnsen scores were significantly higher for the preventive (all p<0.0001) and curative (all p<0.01) groups than for the other groups. Furthermore, the tissue structure status was significantly better in the preventive group compared to the curative group (p<0.01) (Figure 4).
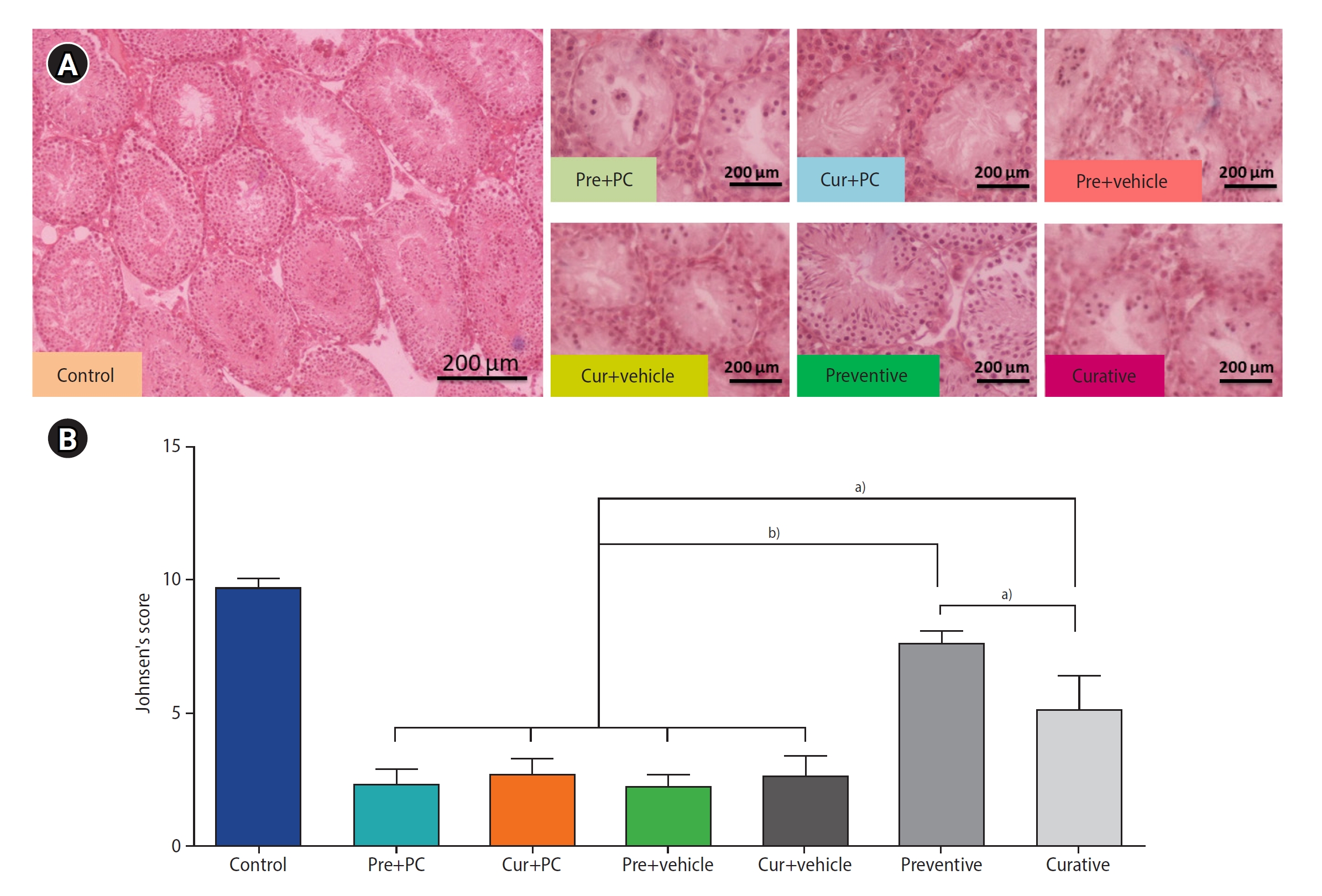
Effects of caffeine on histological structure in mice with induced chronic scrotal hyperthermia. (A) Histological photomicrographs under hematoxylin and eosin staining. (B) Comparison of histological structure across groups based on Johnsen score. Data are presented as mean±standard deviation. Pre, preventive; PC, positive control; Cur, curative. a)p<0.01; b)p<0.0001.
2) Testicular volume
A significantly greater testicular volume was observed in both the preventive (all p<0.0001) and curative (all p<0.05) groups than in the other treatment groups. Additionally, regarding the two caffeine-treated groups, the preventive group exhibited a significantly larger testicular volume than the curative group (p<0.01) (Figure 5A).
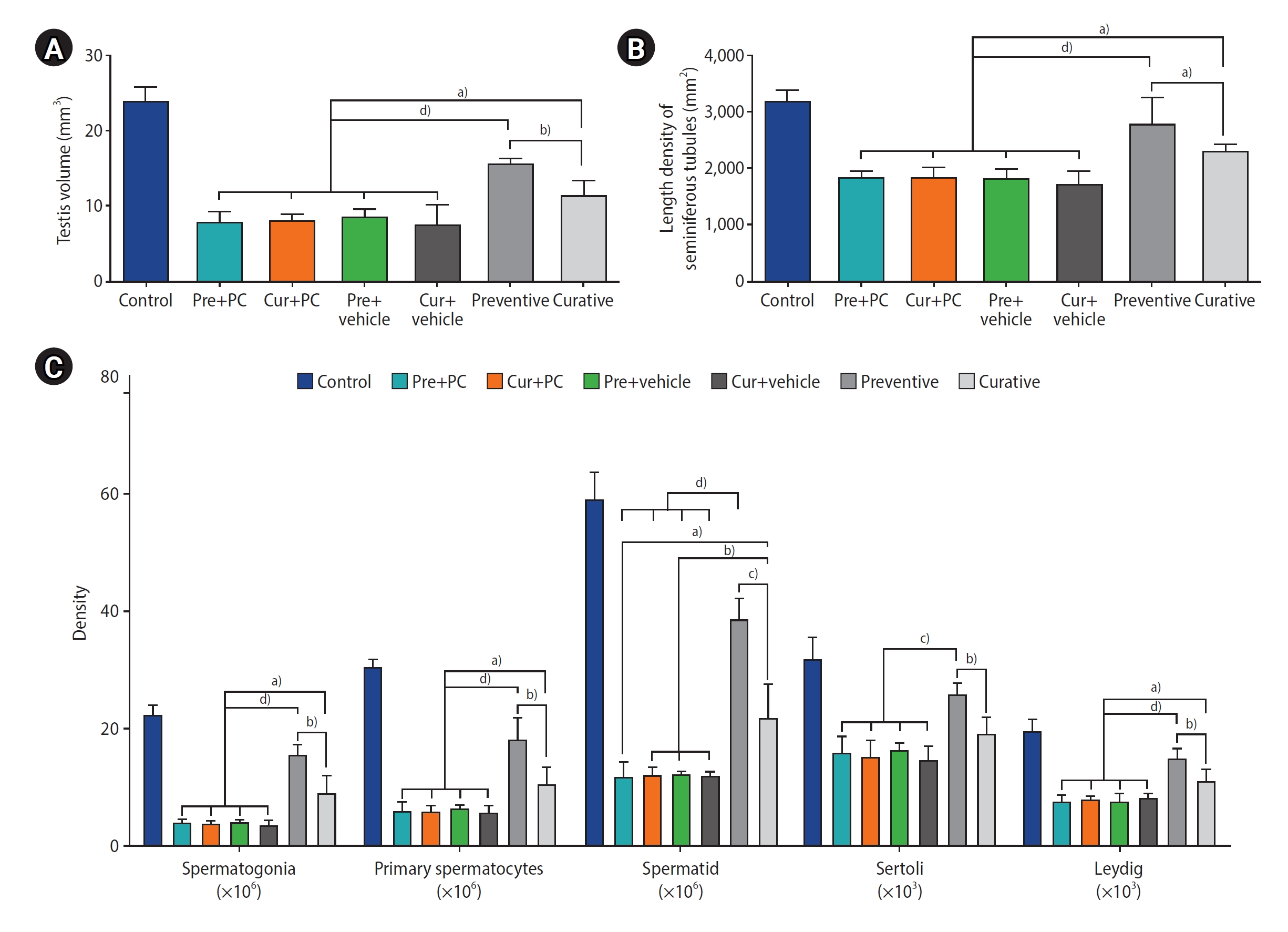
Effects of caffeine on stereological parameters in mice with induced chronic scrotal hyperthermia. (A) Testicular volumes calculated using the Cavalieri method. (B) Length density of the seminiferous tubules. (C) Numerical density of spermatogonia, primary spermatocytes, spermatids, Sertoli cells, and Leydig cells. Data are presented as mean±standard deviation. Pre, preventive; PC, positive control; Cur, curative. a)p<0.05; b)p<0.01; c)p<0.001; d)p<0.0001.
3) Numerical cell density and length density of the seminiferous tubules
The quantitative outcomes of the stereological evaluations are presented in Figure 5B, 5C. In terms of spermatogonia, the numerical density of cells was significantly elevated in both the preventive (all p<0.0001) and curative (all p<0.05) groups relative to the other groups. Furthermore, regarding the two caffeine-treated groups, the preventive group had a significantly higher density of spermatogonia than the curative group (p<0.01).
When comparing primary spermatocyte density between groups, both the preventive group (all p<0.0001) and the curative group (all p<0.05) had significantly higher numbers of cells than the other groups. Furthermore, the density of primary spermatocytes in the preventive group was significantly greater than that of the curative group (p<0.01).
Evaluation of the spermatids revealed that their numerical density was significantly higher in the preventive group relative to the Pre+PC, Cur+PC, Pre+vehicle, and Cur+vehicle groups (all p<0.0001), as well as the curative group (p<0.001). Moreover, the curative group exhibited a significantly higher cell count compared to the Pre+PC, Cur+PC, Pre+vehicle, and Cur+vehicle groups (p<0.05, p<0.01, p<0.01, and p<0.01, respectively).
The numerical density of Sertoli cells in the preventive group was significantly elevated compared to the Pre+PC, Cur+PC, Pre+vehicle, and Cur+vehicle groups (all p<0.001), as well as the curative (p<0.01) group.
In comparing the numerical density of Leydig cells across groups, both the preventive group (all p<0.0001) and the curative group (all p<0.05) had significantly more cells than the other groups. Additionally, regarding the two caffeine-treated groups, the preventive group had a significantly higher Leydig cell density than the curative group (p<0.01).
A comparison of the length density of the seminiferous tubules revealed that the preventive group had a significantly higher number of tubules than the Pre+PC, Cur+PC, Pre+vehicle, Cur+vehicle (all p<0.0001) groups, as well as the curative (p<0.05) group. Furthermore, the curative group exhibited a significantly higher length density of seminiferous tubules compared to the Pre+PC, Cur+PC, Pre+vehicle, and Cur+vehicle groups (all p<0.05).
4. Administration of caffeine inhibits testicular cell apoptosis in mice with induced chronic SHT
Figure 6A presents photomicrographs of immunostaining against the caspase-3 antibody. An examination of the apoptotic cell density in the testicular tissues indicated that the density of apoptotic cells in both the preventive (all p<0.0001) and curative (all p<0.05) groups was significantly lower relative to the other groups. Moreover, the preventive group exhibited a significantly lower degree of apoptotic cells than the curative group (p<0.01) (Figure 6B).

Effects of caffeine on cell apoptosis in mice with induced chronic scrotal hyperthermia. (A) Representative micrographs from immunostaining against caspase 3 protein in the testicular tissue. Caspase 3-positive cells are represented by dark brown cytoplasm. (B) Quantitative analysis of caspase 3-positive cells. Data are presented as mean±standard deviation. Pre, preventive; PC, positive control; Cur, curative. a)p<0.05; b)p<0.01; c)p<0.0001.
5. Administration of caffeine modulates testicular oxidative biomarkers in mice with induced chronic SHT
Figure 7A presents an analysis of oxidative biomarkers in the testicular tissue, focusing on both oxidative (MDA) and antioxidative (GSH, SOD, and CAT) factors. Both the preventive (all p<0.0001) and curative (all p<0.01) groups exhibited significantly lower MDA levels than the other experimental groups. Furthermore, the preventive group demonstrated significantly diminished MDA levels relative to the curative group (p<0.01).
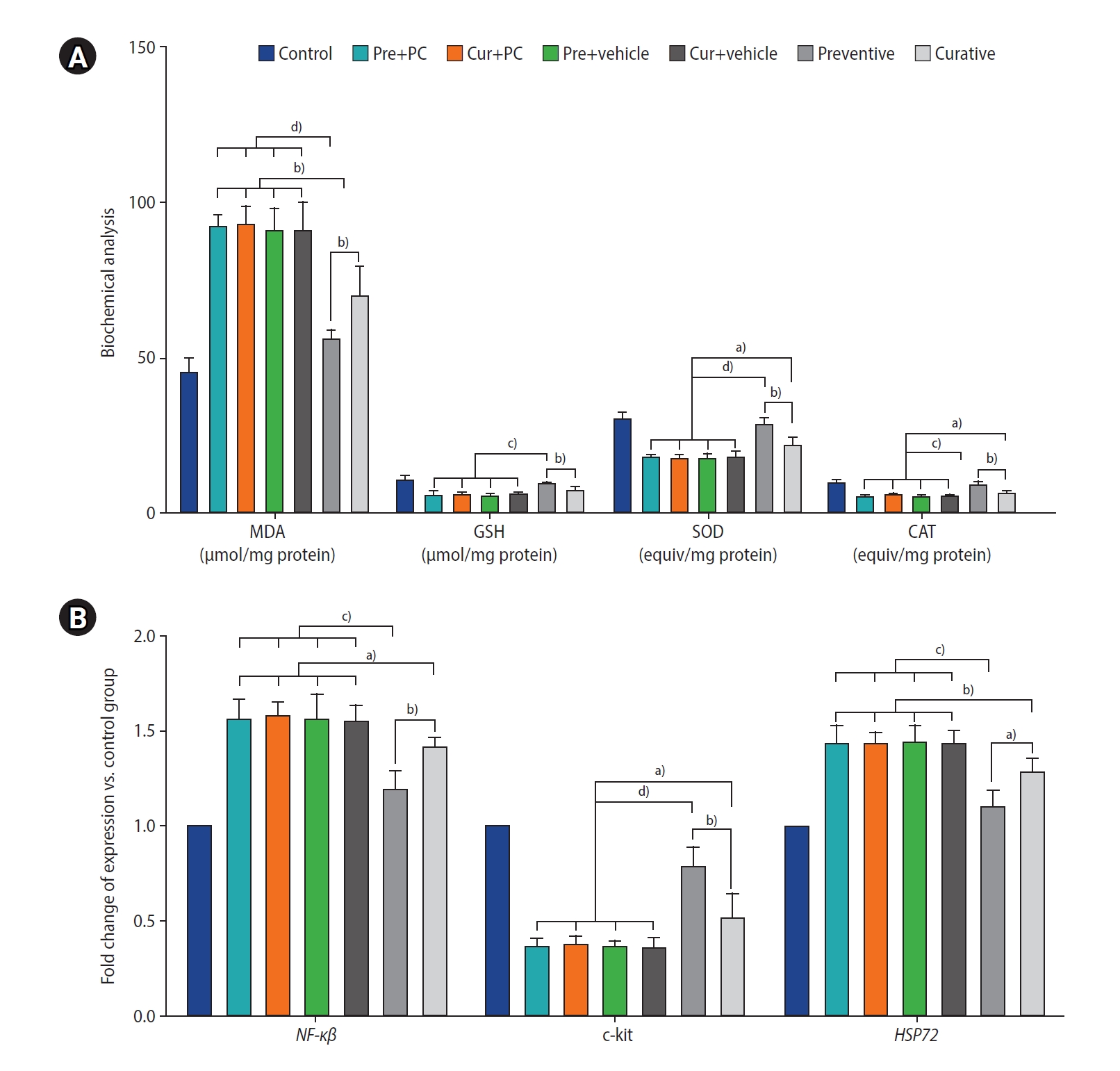
Effects of caffeine on testicular oxidative biomarkers and gene expression in mice with induced chronic scrotal hyperthermia. (A) Concentrations of oxidant (malondialdehyde [MDA]) and antioxidant (glutathione [GSH], superoxide dismutase [SOD], and catalase [CAT]) factors in the testicular tissues were evaluated using the biochemical method. (B) The quantities of transcripts for nuclear factor kappa beta (NF-κβ) (a gene involved in inflammation), as well as c-kit and heat shock protein 72 (HSP72) (involved in spermatogonial differentiation, proliferation, and resistance to oxidative stress), were analyzed using quantitative real-time polymerase chain reaction. Data are presented as mean±standard deviation. Pre, preventive; PC, positive control; Cur, curative. a)p<0.05; b)p<0.01; c)p<0.001; d)p<0.0001.
Regarding levels of antioxidative biomarkers, the preventive group had significantly higher levels of GSH, SOD, and CAT than the Pre+PC, Cur+PC, Pre+vehicle, Cur+vehicle, and curative groups (p<0.001, p<0.001, p<0.001, p<0.001, and p<0.01, respectively for GSH; p<0.0001, p<0.0001, p<0.0001, p<0.0001, and p<0.01, respectively for SOD; and p<0.001, p<0.001, p<0.001, p<0.001, and p<0.01, respectively for CAT). Furthermore, relative to the Pre+PC, Cur+PC, Pre+vehicle, and Cur+vehicle groups, the curative group exhibited significantly higher levels of both SOD and CAT (all p<0.05).
6. Administration of caffeine affected testicular gene expression in mice with induced chronic SHT
The levels of NF-κβ gene expression were significantly lower in both the preventive (all p<0.001) and curative (all p<0.05) groups relative to the other experimental groups. Moreover, the level of NF-κβ gene expression was found to be lower in the preventive than in the curative group (p<0.01) (Figure 7B).
Regarding the c-kit gene, both the preventive group (all p<0.0001) and the curative group (all p<0.05) exhibited significantly elevated expression levels relative to the other groups. Furthermore, when compared to the curative group, the preventive group demonstrated significantly greater expression of c-kit (p<0.01).
Finally, the level of HSP72 gene expression was markedly downregulated in both the preventive (all p<0.001) and curative (all p<0.01) groups when compared to the other groups. Moreover, the preventive group exhibited significantly lower HSP72 expression than the curative group (p<0.05).
Discussion
Testicular hyperthermia is widely recognized as a primary cause of male infertility, associated with an overproduction of reactive oxygen species (ROS) and the initiation of sperm apoptosis in the testes [42]. In recent years, increasing attention has been paid to the detrimental impact of oxidative stress on male infertility, as well as the potential benefits of antioxidant supplements in enhancing semen parameters among men with infertility [43]. The administration of antioxidant supplements may enhance the body’s capacity to inhibit the oxidative chain reaction, thereby improving the spermatogenic process [44]. In the present study, we observed that all evaluated sperm characteristics, including count, viability, motility, and morphology, were significantly superior in the preventive and curative groups compared to the other groups. Numerous studies have highlighted the anti-inflammatory, anticancer, and antioxidant properties of caffeine, even suggesting its potential as a preventative measure against the effects of various drugs and environmental factors on fertility and testicular tissue [15,31,45,46]. Our findings indicate that caffeine mitigates the tissue damage induced by chronic hyperthermia in testicular tissue. This includes key genes involved in germ cell differentiation and inflammation (such as NF-κβ, c-kit, and HSP72), oxidative biomarkers, and sperm parameters, as well as testicular morphology and stereology. The observed protective effect can be attributed to the antioxidant properties of caffeine.
Research has demonstrated that carbohydrates, particularly glucose, serve as the primary and essential energy source for mitotic cellular division. The blood-testicular barrier is the main pathway for glucose transfer to the seminiferous tubules [47,48]. Hyperthermia results in a decrease in testosterone synthesis by Leydig cells and a reduction in the functionality of Sertoli cells [49,50]. Elevated levels of oxidative stress, induced by testicular hyperthermia, adversely impact sperm parameters, egg fusion, sperm, and sperm epigenetic modifications [51,52]. The overproduction of ROS can trigger oxidative stress, leading to impaired spermatogenesis, increased morphological damage, decreased motility, and impaired sperm-oocyte binding [5,53]. Caffeine has been found to mitigate oxidative stress and elevated lipid peroxidation levels in sperm and testicular cell membranes. It does this by enhancing the activity of antioxidant enzymes such as CAT, SOD, and GSH peroxidase, which in turn leads to a decrease in sperm motility and spermatozoon malformation [15]. Furthermore, caffeine has demonstrated its capacity to protect DNA by clearing ROS.
Caffeine has been reported to increase sperm activity and preserve fertility via a cyclic adenosine monophosphate (cAMP)-independent pathway, specifically through a mechanism that relies on protein tyrosine phosphorylation and calcium [54]. Li et al. [55] provided evidence that a single exposure of the scrotum to heat at 42 °C for 25 minutes resulted in an elevated level of MDA, a decrease in antioxidant enzymes (particularly SOD), an increase in germ cell apoptosis through the caspase-3 pathway, and upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2). These findings align with several results of our study.
Caffeine inhibits cyclic nucleotide phosphodiesterase, leading to an increase in intracellular cAMP and intracellular calcium ion levels. These changes enhance sperm viability by inhibiting the caspase-3 apoptotic pathway and preserving the normal structure of the testis [56], potentially helping to explain the findings of our study. Additionally, previous research indicated that 0.4 mg/mL of caffeine enhances sperm motility and vitality in vitro, while also boosting the fertilization capacity and the rate of embryo formation in mice [57].
Given that NF-κB plays a crucial role in numerous cellular functions—particularly proliferation and apoptosis—and also regulates certain pro-inflammatory cytokines such as interleukin 1 beta and tumor necrosis factor-alpha, it is plausible that caffeine may regulate inflammation and oxidative stress levels. This regulation could occur through indirect modulation of these cytokines, subsequently enhancing sperm parameters [58,59].
Alterations in membrane proteins and phospholipids can lead to enhanced sperm motility and improved acrosome reaction [60]. Caffeine plays a role in this process by inhibiting cyclic nucleotide phosphodiesterase. This inhibition results in an increase in intracellular cAMP, subsequently inducing the acrosome reaction [56].
Moreover, proteoglycans, including chondroitin and heparin, are crucial for initiating sperm capacitation and the acrosome reaction, signifying a key role of Sertoli cells [61]. Caffeine enhances the production of these proteoglycans and testosterone, which is secreted by Leydig cells. Subsequently, testosterone activates mitogen-activated protein kinase in Sertoli cells [62]. This signaling pathway could partially explain the results of our study, which include an increase in germ cell viability and the upregulation of proliferative factors such as c-kit and HSP72.
Conversely, nitric oxide is a free radical and a messenger that regulates protein phosphorylation. This modulation affects various physiological processes, particularly spermatogenesis, which exhibits overexpression following hyperthermia [28,63].
The progression of sperm capacitation necessitates an increase in the intracellular concentration of cyclic guanosine monophosphate (cGMP) [64]. Given that cGMP promotes sperm capacitation and the acrosome reaction, caffeine can enhance the acrosome reaction and improve sperm viability by increasing cGMP concentration [65]. Furthermore, caffeine activates protein kinase C [66], a fundamental player in the signaling pathway that contributes to sperm maturation, viability, and motility [67].
The primary mechanism of action for caffeine appears to be the antagonism of adenosine receptors [68]. Given the similar molecular structures of caffeine and adenosine, both of which possess a double-bond ring structure, caffeine can bind to adenosine receptor sites [69]. It has long been suggested that adenosine and its antagonists may influence the male reproductive system [70]. Multiple studies have demonstrated the presence of adenosine receptors in Sertoli cells [71,72] and have shown that these cells can be modulated by adenosine and its analog compounds. Sertoli cells play a crucial role in the functional development of the testis and, consequently, in the expression of the male phenotype [73,74]. They also actively metabolize several substrates, such as glucose, to ensure a steady supply of lactate to the developing germ cells [75]. Therefore, the overall metabolic functioning of Sertoli cells is critical for normal spermatogenesis. Several studies have indicated that caffeine can stimulate Sertoli cells to increase testosterone production [15,76,77]. In line with these findings, our study results demonstrated significantly elevated testosterone levels in the group that received caffeine compared to the other groups.
In contrast, Sertoli cells are known to produce lactate at high rates. Any disruption in related processes could potentially result in increased levels of oxidative stress, which may subsequently lead to male subfertility or infertility [78]. Intriguingly, caffeine has been identified as a protective agent against cellular damage, offering beneficial antioxidant effects [79]. The results of our study similarly demonstrated a significant reduction in the oxidative stress level within the testicular tissue of the groups that received caffeine. These findings align with the results of previous studies.
In conclusion, our findings indicate that SHT leads to a decrease in sperm parameters and testicular dysfunction, primarily through the induction of inflammation, oxidative stress, and apoptosis. Nevertheless, caffeine, which possesses potent antioxidant and anti-apoptotic properties, has the potential to enhance sperm parameters and address infertility issues associated with heat-induced oxidative stress.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: AR, DN. Data curation: AR, HM, FB. Formal analysis: AR, OG. Funding acquisition: AR, DN. Methodology: AR, HM, DN, AG. Project administration: AR, DN, AG. Visualization: AR, DN, AG. Writing-original draft: AR, DN, AG. Writing-review & editing: AR, DN, AG.
Acknowledgements
The authors express their gratitude for the support of Mazandaran University of Medical Sciences in Sari, Iran.